Blogs
PDK-1 overview
Protein 3-phosphoinositide-dependent protein kinase-1 (PDK-1)
As a member of AGC kinases family, PDK-1, a protein of 556 amino acids, is composed of serine and threonine kinases. The catalytic domain of these serine and threonine kinases show a sequential similarity with cAMP-dependent protein kinase 1 (PKA), cGMP-dependent protein kinase (PKG) and protein kinase C (PKC). There are two phosphorylation sites that many AGC kinases and they are to regulate the activity of AGC kinases. The one which is located within the kinase domain is called activation loop, while the other one named hydrophobic motif is located in a region which is adjacent to the catalytic domain. The enzymatic full activation is triggered by phosphorylation of activation loop and hydrophobic motif which is catalyzed by an autophosphorylation reaction. n addition, PDK-1 kinase also has a PH domain. The PH domain is used for mainly interacting with phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate that is essential in localization and activation of some of membrane associated PDK-1's substrates such as AKT. The kinase domain has three ligand binding sites, namely the substrate binding site, the ATP binding site, and the docking site which is also known as PIF pocket. The PIF pocket interacts and binds some PDK-1 substrates such as S6K and Protein kinase C . Several small molecule allosteric activators of PDK-1 in previous studies were shown to inhibit the activation of substrates selectively. Instead of binding to the active site, these small molecules enable PDK-1 to activate other substrates which do not require docking site interaction. So far, there is no well-defined inhibitor for PDK-1. One of the most important substrate of PDK-1 is AKT. The activation of AKT requires a proper orientation of the kinase and PH domains of PDK-1 and AKT at the membrane. Many proteins that interact with PDK-1 via a hydrophobic motif named PDK-1 interacting fragment (PIF). This fragment lies in a conserved Phe-Xaa-Xaa-Phe/Tyr-Ser/Thr-Phe/Tyr sequence where phosphorylation occurs in serine or threonine. Although PDK-1 is the only member of the AGC kinase family that lacks the hydrophobic motif, it has a hydrophobic pocket that is also called PDK1 interacting fragment pocket (PIF pocket). This plays an important role in the interaction between PDK-1 and the hydrophobic motif of the targeted protein kinases. PIF pocket binds the PIF hydrophobic motif via a docking site, which promotes the phosphorylation of the targeted kinase at the activation loop. PDK-1 is significant for the activation of many other AGC kinases including PKC , S6K , SGK and AKT /PKB via phosphorylation. An important role for PDK-1 is in the signaling pathways activated by several growth factors and hormones including insulin signaling.
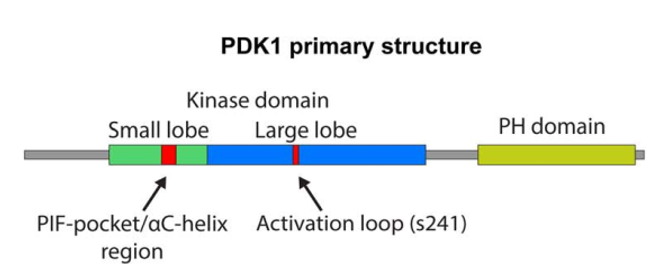
Figure 2. PDK-1 primary structure
PDK-1 signaling pathway
-
PDK-1 signaling cascade
Class IA phosphoinositide 3 kinases (PI3Ks) which is composed of p110α–p85, p110β–p85 and p110δ–p85 are recruited to the membrane by direct interaction of the p85 subunit with the activated receptors such as platelet derived growth factor receptor or by the association with adaptor proteins interacted with the receptors like insulin receptor substrate 1 after the stimulation by growth factor and subsequent activation of receptor tyrosine kinases (RTKs). Next, the activated p110 catalytic subunit converts phosphatidylinositol 4,5 bisphosphate (PtdIns(4,5)P2) to phosphatidylinositol 3,4,5 trisphosphate (PtdIns(3,4,5)P3) at the membrane, which offers docking sites for downstream signaling proteins, namely putative 3 phosphoinositide dependent kinase 1 (PDK-1) and serine–threonine protein kinase AKT (also known as protein kinase B). PDK-1 phosphorylates AKT, which activates AKT. This activation initiates a wide range of downstream signaling events. The interaction of G protein coupled receptors (GPCRs) and Gβγ subunit of trimeric G proteins can lead to the direct activation of the class IB PI3K (p110γ–p101). GPCR can activate the p110β and p110δ subunits as well. At the same time, PTEN (phosphatase and tensin homologue) antagonizes the PI3K action through the dephosphorylation of PtdIns(3,4,5)P3.
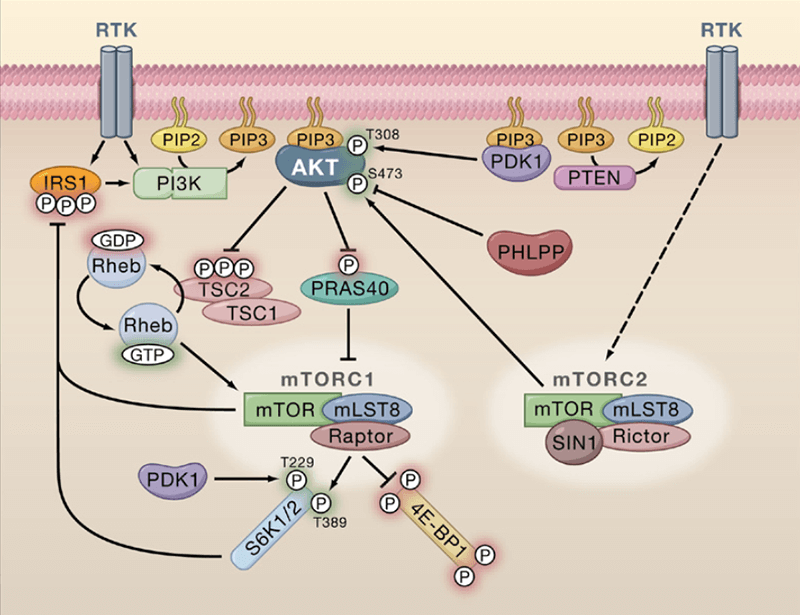
Figure 3. PDK-1 signaling cascade
-
Downstream signaling
In previous studies, PDK-1 has shown its function to activate many other members of AGC kinase family like p70S6K, SGK, p90RSK and the members of PKC family via phosphorylation. Different from some mechanisms of other kinases cascades that it is linear and consecutive activation events, the phosphorylation through PDK-1 requires to be coupled with another convergent signal. For example, the activation of AKT requires a proper orientation of the kinase and PH domains of PDK-1 and AKT at the membrane. At the same time, other substrates need the phosphorylation of their hydrophobic motif by other kinases. In addition to the conventional substrates among AGC kinase family, PDK-1 also show the phosphorylation ability to other proteins. Polo-like kinase 1, p21- activated kinase (PAK) and adhesions proteins like β3 integrin are also targets of PDK-1. In addition, some downstream effectors can also be regulated by PDK-1 via kinase independent mechanisms, including myotonic dystrophy kinase-related CDC42-binding kinase alpha (MRCKα) and Rho-associated protein kinase 1 (ROCK1). At the first time that PDK-1 was discovered, scientists found that it was for phosphorylating the AKT activation loop at residue Thr308, which was important for the activation of AKT. There would be further phosphorylation of AKT at residue Thr308 that was dependent on PtdIns(3,4,5)P3. Even if AKT is the primary target of PDK-1, plenty of other kinases are also downstream targets. For instance, serum glucocorticoid-dependent kinase (SGK) which is one member of AGC family kinases, p90 ribosomal protein S6 kinase (RSK), p70 ribosomal protein S6 kinases (S6K) and atypical protein kinase C (PKC) isoforms are known to be a direct target of PDK-1. In these interactions, PDK-1 phosphorylates specific serine/threonine residues of target proteins' activation loop. Therefore, PDK-1 has been regarded as the master regulator in AGC-related signaling pathways and plays a significant role in controlling cell motility proliferation and survival.
-
Pathway regulation
The regulation of signaling activated by PDK-1 involves different mechanisms
Since the PH domain of PDK-1 interacts with phosphatidylinositol 3,4-bisphosphate PtdIns(3,4)P2, PtdIns(4,5)P2 and phosphor-nositides PtdIns(3,4,5)P3 with high affinity, PDK-1 is anchored at the plasma membrane. This close association shows a potential PI3K-dependent PDK1 membrane translocation. Even though the membrane localization has been greatly studied, the dynamic mechanism is still not well-described. Especially, with the stimulation of growth factors, whether PDK-1 is constitutively localized to the plasma membrane or it trans-locates to the plasma membrane is unknown yet. The mechanism of PDK-1 membrane localization is important for the phosphorylation of AKT at the residue Thr308. The PI3K activation induces the membrane translocation of AKT, which results in the co-localization of PDK-1 at the plasma membrane. The membrane recruitment of AKT directly leads to the conformational changes and these changes promote the phosphorylation of AKT at Thr308 by PDK-1. When the PH domain of AKT is mutated, the membrane recruitment of AKT is severely destroyed, which leads to the significant decrease of AKT phosphorylation by PDK-1. The deletion of the PDK1-PH domain significantly decreases AKT phosphorylation as well. PDK-1 also shows a manner of dimeric conformation via PH domain interaction of two PDK-1 monomers. This interaction plays an important role in the phosphorylation of AKT. If the formation of the homodimer is inhibited by destroying the PH domain, the more active form monomeric PDK-1 is released, which promote the phosphorylation of AKT. -
Relationship with diseases
The hyperactivation of the PI3K/PDK-1/AKT pathway has a close association with many human cancers. PDK-1 is of paramount importance in breast cancer initiation and progress. The bigger copy number of PDK-1 is universal among patients with breast cancer. This high level of PDK-1 is related to the activation of PI3K pathway. At the same time, the mutant PI3K class 1 p110 seems to accumulate with the increasing PDK-1 copy number. The overexpression is shown to promote the transformation of mammary epithelial cells. More than 45% patients with acute myeloid leukemia (AML) have been reported that their PDK-1 level was higher. Compared to primary tumors, the locus where PDK-1 gene locates is more amplified in lymph node metastases and castration-resistant prostate cancer. At the same time, the expression level of PDK-1 protein is much higher in tumor cells compared to adjacent non-cancerous tissues in esophageal squamous cell carcinoma. In gastric carcinoma, both mRNA and protein expression level of PDK-1 are higher in tumor samples than those in adjacent normal tissues. Shorter survival has also been shown in patients with higher PDK-1 expression. The similar situation was observed in hepatocellular carcinoma. Many studies have shown that the overexpression of PDK-1 contributes a lot to a variety of cancers and is highly associated with advanced tumor stages.

Figure 4. PDK-1 alterations in cancer
However, whether PDK-1 is crucial to tumorigenesis or is just required in later stages is still not well-defined. Some studies suggested that PI3K can promote cancer via both AKT-dependent and AKT-independent regulation. Not like other AGC kinases, the phosphorylation on the activation loop of PDK-1 at serine 241, instead of by other kinase proteins, is catalyzed by itself. The de-phosphorylation of phospho-serine 241 is little efficient since phosphatases have no access to it. In such case, PDK1 has been considered constitutively active. In addition, homo-dimerization is highly related to the regulation of PDK-1 activity. The drugs targeted at PDK-1 are promising in therapies of many related cancers.
References
- Liu P, Cheng H, et al. Targeting the phosphoinositide 3 kinase pathway in cancer. Nature Reviews Drug Discovery. 2009 Aug 5;8(4):627-44.
- Gagliardi PA, Puliafito A, Primo L. PDK1: At the crossroad of cancer signaling pathways. Seminars in Cancer Biology. 2017 May 1;28:27-35.
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261-74.
- Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017 Apr 20;169(3):381-405.
- Raimondi C, Falasca M. Targeting PDK1 in cancer. Current Medical Chemistry. 2011 Jul 1;18(7): 2763-69.
Posted in: Health
Topics:
signaling pathway
Be the first person to like this.






